ATLANTA-- BUSINESS WIRE-- St. The CardioMEMS HF System is indicated for wirelessly measuring and monitoring pulmonary artery PA pressure and heart rate in New York Heart Association NYHA Class III heart failure patients who.
 St Jude Medical And Cardiomems Announce Fda Approval Of The Cardiomems Heart Failure Management System Business Wire
St Jude Medical And Cardiomems Announce Fda Approval Of The Cardiomems Heart Failure Management System Business Wire
Jude Medical and CardioMEMS are not the only two entities celebrating the FDA approval of the worlds first implantable wireless pulmonary artery pressure sensing.

Cardiomems fda approval. NYSESTJ a global medical device company and CardioMEMS today announced US. December 8 2011 October 9 2013. March 20 2019.
Jude MedicalAbbott in the after-market setting is associated with decreased heart-failure hospitalizations across all patient sex race and ejection fraction categories with few device-related complications at 1 year according to a postapproval study. Approval for the cardiomems hf system which includes the cm2000 implantable pa sensormonitor and transvenous catheter delivery system the. A PMA is an application submitted to FDA to request approval to market.
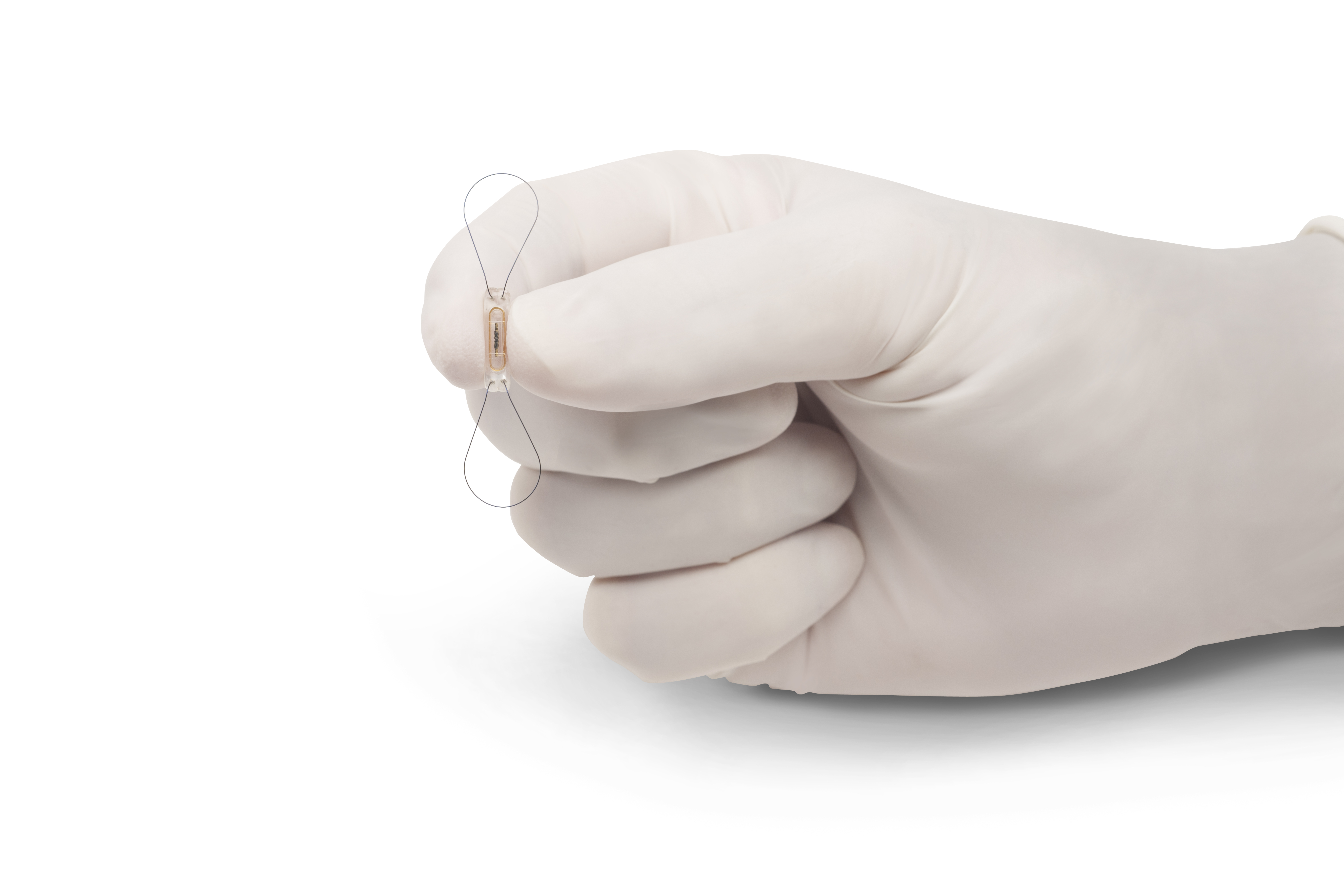
Premarket Approval PMA is the most stringent type of device marketing application required by FDA. ATLANTA Arcapita Ventures announced today that CardioMEMS Inc. May 28 2014The US Food and Drug Administration FDA announced that it has approved the CardioMEMS HF system CardioMEMS Inc that measures the pulmonary artery PA pressures and heart rates of patients with New York Heart Association NYHA class III heart failure who have been hospitalized for heart failure in the previous year.
The FDA recent approval of a heart failure monitoring device will allow physicians to check up on patients health remotely. Premarket Approval Application PMA Number. CardioMEMS HF System for Pulmonary Pressure Monitoring in Heart Failure Patients Approved by FDA VIDEO May 29th 2014 Medgadget Editors Cardiac Surgery Cardiology Radiology.
The FDA approval was based in part on CardioMEMS 550-patient clinical trial which found that at 6 months 986 of patients had no device-related safety. FDA regulators today approved a 1st-of-its-kind implantable heart monitor CardioMEMS Champion HF technology for remote patient monitoring spurring St. Intel Free Press CC BY-SA 20 The Food and Drug Administration FDA announced Thursday its approval for the first wireless implantable device known as CardioMEMS HF System designed to monitor the health of heart failure patients remotely.
FDA Food and Drug Administration approval for its Heart Failure Management System. Food and Drug Administration FDA approval of the. Applicants Name and Address.
The FDA approval of St. Jude Medical and CardioMEMS implantable wireless heart monitoring device has gotten the medtech world excited and that includes a competitor. FDA has completed its review of your premarket approval application PMA for the CardioMEMS HF System which includes the CM2000 implantable PA.
FDA Food and Drug Administration approval for its Heart Failure Management System. The approval is based upon the results of the CHAMPION CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients clinical trial which demonstrated a 28 reduction in. NEW ORLEANS LAThe performance of the CardioMEMS HF System St.
Dates of Panel Recommendation. ATLANTA May 28 2014 PRNewswire -- Arcapita Ventures announced today that CardioMEMS Inc. Jude initially took a 19 percent stake and the option to buy the remainder of CardioMEMS for 60 million in September 2010.
47 Zeilen Medical Devices Cleared or Approved by FDA in 2019.