The CardioMems device provides numerous benefits to patients like you with Class III congestive heart failure. The CardioMEMS system has been heralded as a new approach to heart failure pinpointing worsening congestive heart failure CHF earlier to allow physicians to treat patients more effectively.
 Major Components Of The Cardiomems Hf System Left And Anatomical Download Scientific Diagram
Major Components Of The Cardiomems Hf System Left And Anatomical Download Scientific Diagram
Metallurgical Engineering Materials Science various locations MEMS.

What does cardiomems stand for. The approval makes the core technology - pulmonary artery pressure measurement - which is very well known and regarded in clinical circles - available to monitor congestive heart failure patients at home which many consider a clinical breakthrough. The sensor is implanted during a minimally invasive procedure is designed for lifetime use and does not need a battery or replaceable parts. WHAT IS HEART FAILURE.
The purpose of the CHAMPION CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients clinical trial was to show that patients managed to PA pressures using the CardioMEMS HF System have fewer heart failure hospitalizations than those managed by standard of care alone. Voice calls are placed on the hospitals VoIP system through the hospitals PBX and Wi-Fi system. CardioMEMS a New Option for Monitoring Heart Failure.
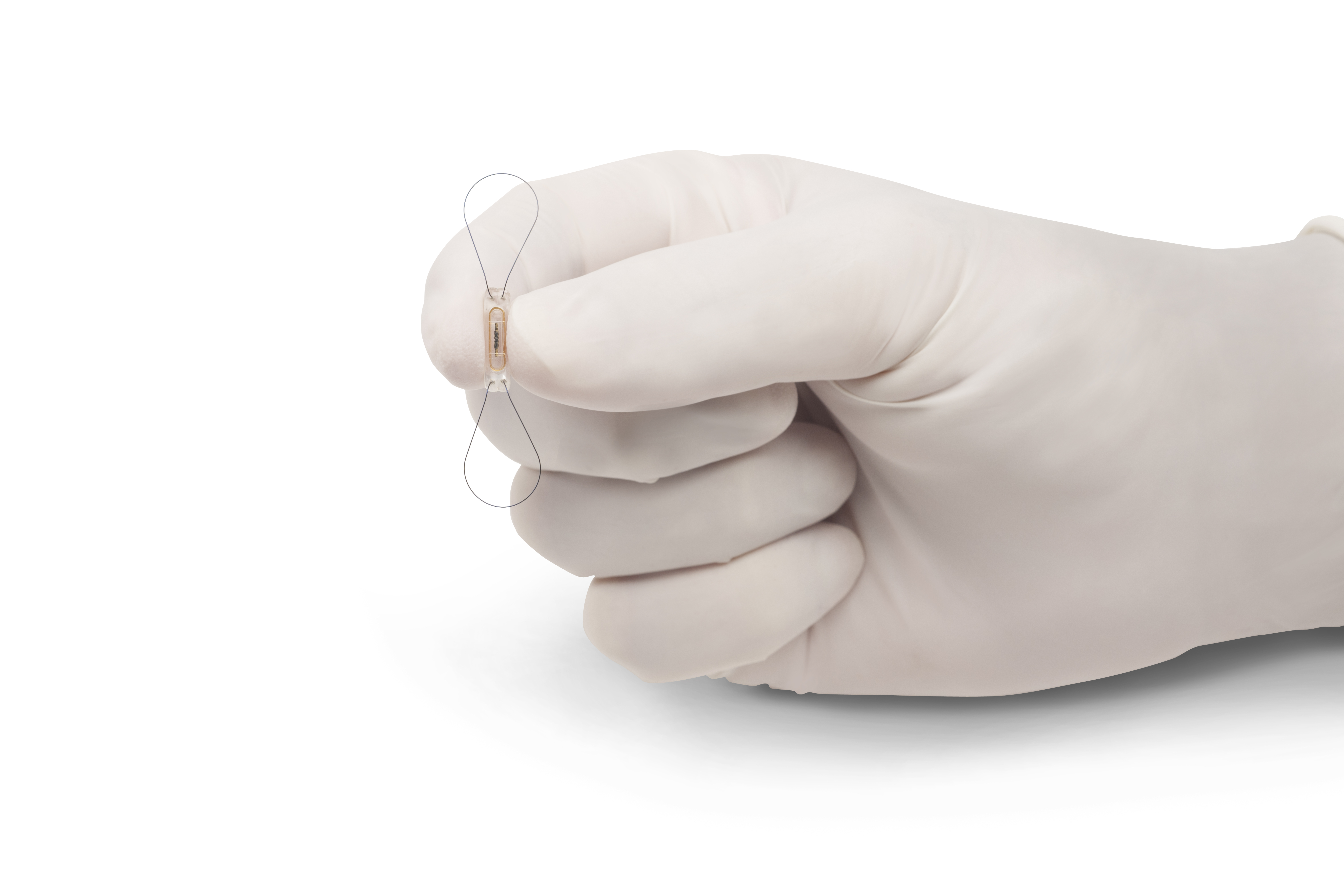
The CardioMEMS HF System consists of a small sensor size of a paper clip that is placed directly in the pulmonary artery as well as a home monitoring unit that you can use to take daily measurements. Their miniature wireless sensors can be implanted using minimally invasive. Its the first and only FDA-approved heart failure monitoring and tracking device clinically proven to reduce hospitalizations in patients and improve quality of.
Significant HF progression over a period of days is known as acute decompensation and leads to hospitalization. Or that your heart muscle is too thick and does not relax enough between beats to allow it to fill with blood. They volunteer their time to share their story with others who have heart failure.
The CardioMEMS HF system is the worlds first implantable wireless pulmonary artery pressure sensing device for heart failure patients. 3 Table of Contents. Their technology platform is designed to improve the management of severe chronic cardiovascular diseases such as heart failure and aneurysms.
Voice calls alarm notifications and text messaging. Permanently implanted in the distal pulmonary artery via a safe right heart catheterization procedure the sensor measures changes in pulmonary artery pressure. Accuracy of the CardioMEMS HF System is slightly affected by large changes in elevation between the initial baseline calibration and subsequent measurements.
Internal jugular IJ subclavian external jugular axillary cephalic and femoral. Directly measuring pulmonary artery pressure PA pressure via a procedure called a right-heart catheterization is a standard practice for managing worsening heart failure in patients who have been hospitalized. These changes are a surrogate measure for fluid retention in the lungs caused by worsening heart failure.
At high levels of PEEP over about 15 cm H 2 O CVP increases as the cardiac output is depressed because of impaired right ventricular output. 2mm Hg 305 meters elevation change. Talk to an ambassador living with a CardioMEMS HF System from Abbott CardioMEMS HF System Ambassadors and their caregivers understand firsthand the journey of heart failure.
But now a new review from a non-profit research organization has suggested that CardioMEMS is too costly in light of its clinical benefit. If you are a patient who has been hospitalized with heart failure within the past year or if you are a physician caring for a patient with this condition please call 631 444-9746 option 4 to discuss eligibility for the CardioMEMS device or to receive more information. Common locations include the following veins.
Watch some of their stories on our website or call 1-855-4-CMEMSHF to speak with an. Accuracy of the CardioMEMS HF System is affected by a change in body temperature -1mm HgΔºC. Medieval and Early Modern Studies various schools MEMS.
The CardioMEMS HF System provides pulmonary artery PA pressure remote monitoring using a small sensor. The application includes three components. The CardioMEMS HF System from CardioMEMS Atlanta GA consists of an implantable battery-free sensor that is implanted into the distal.
Since heart failure is a chronic disease most patients do not remain in the hospital for extended periods of time. Browse and search thousands of Cardiology Abbreviations and acronyms in our comprehensive reference resource. Heart failure can make you.
The alarm notifications are received through integration with hospital systems and devices. CardioMEMS is a medical device company that has developed and is commercializing a proprietary wireless sensing and communication technology for the human body. Voalte One is a clinical communication smartphone application.
The CardioMEMS HF System is the first and only FDA-approved heart failure monitoring device proven to significantly reduce hospital admissions and improve quality of life in class III heart failure patients that have been hospitalized in. The CardioMEMS HF System is contraindicated for patients with an inability to take dual antiplatelet or anticoagulants for one month post implant. When the heart is unable to pump enough blood to meet the bodys demands blood pressure within the heart is elevated leading to heart failure HF.