Women are more likely to suffer from insomnia than men. Introducing the Original CES Alpha Stim Therapy Device.
 Dynamics Of The Cranial Electrotherapy Stimulation Devices Market Ces Ultra
Dynamics Of The Cranial Electrotherapy Stimulation Devices Market Ces Ultra
The basics of Cranial Electrotherapy Stimulation CES is a class of non-invasive transcranial pulsed-current stimulation that applies a particular amplitude frequency and waveform to patients.
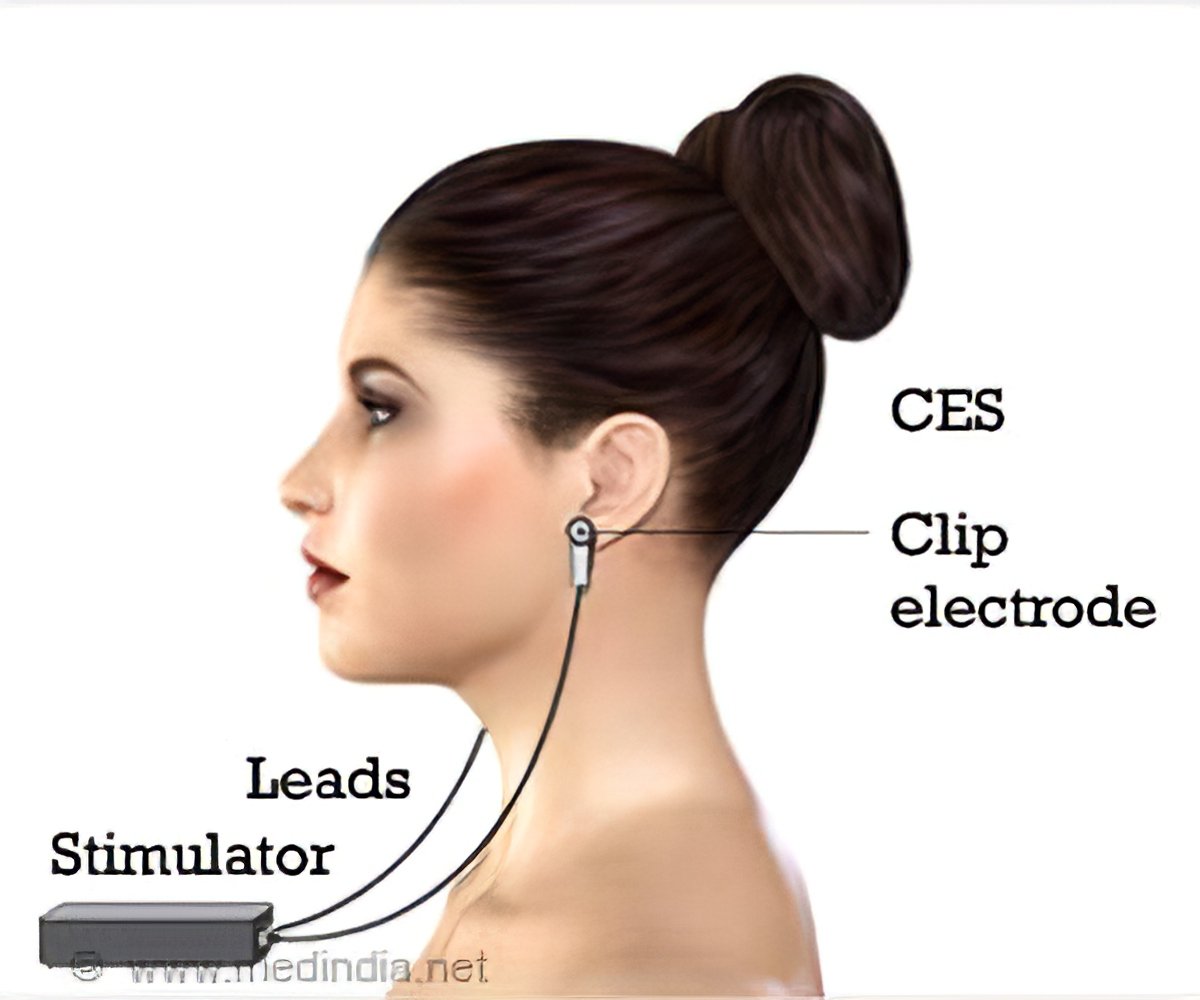
Cranial electrotherapy stimulation ces. Micro-current electrical stimulation also called cranial electrotherapy stimulation CES has been cleared but not officially approved by the US. Its Anti insomnia Anti sleeplessness Anti-anxiety Anti-depression. CES devices have two parts.
Electrosleep treatment an older name for CES came into the USA from Japan in the late 1960s. This is by incorporating CES Cranial Electrotherapy Stimulation with Microcurrent technology and a patented waveform. Free UK delivery on eligible orders.
CES Devices are FDA Cleared for the treatment of Anxiety and Insomnia. Free UK delivery on eligible orders. What is Cranial Electrotherapy Stimulation CES.
Cranial electrotherapy stimulation CES is considered to be a potential treatment for insomnia. The Original CES Alpha Stim Therapy Device is a CES therapy device that stimulates the brain to produce serotonin while lowering the stress hormone known as the cortisol responsible for healthy sleep and mood. As of January 2021 FDA guidlines have changed removing depression clearance from most CES devices.
Food and Drug Administration for. For USA Customers Please note that a licensed healthcare provider must authorize your purchase before we can ship your device. What is cranial electrotherapy stimulation or CES.
Ad Check out our selection order now. Cranial Electrotherapy Stimulation CES is an electromedical modality indicated for the treatment of anxiety depression and insomnia. Ad Check out our selection order now.
This therapy had been originally pioneered in. NeuroCes Cranial Electrotherapy Stimulator has been approved and CE certified as a therapeutic medical device for both clinical use and personal use in the treatment of Depression Anxiety and Insomnia. The Alpha-Stim AID offers a revolutionary clinically proven drug-free and completely safe and natural solution for treating a wide variety of psychological conditions such as anxiety insomnia and depression.
Side effects can include vertigo or dizziness if the stimulation is set too high or you cant tolerate the machine headache approx 1 in 1000 or skin irritation approx 1 in 1500. Cranial electrical stimulation CES is a non-invasive method of applying low-intensity electrical current to the head. Here are answers to some commonly asked questions about CES and Alpha-Stim.
The device she uses is called the Alpha-Stim an iPhone-size. Therefore we studied the effect of CES on sleep efficiency in young healthy women. It is related to but distinct from other forms of transcranial electrical stimulation including electroconvulsive therapy transcranial direct current stimulation tDCS and high-definition transcranial direct current stimulation.
NeuroCes reduces anxiety depression and insomnia symptoms in a spectacular way with no significant side effects. The something else turned out to be cranial electrotherapy stimulation or CES.


