Ocrelizumab sold under the brand name Ocrevus is a pharmaceutical drug for the treatment of multiple sclerosis. South San Francisco CA.
 P115 Safety Of Ocrelizumab In Multiple Sclerosis Updated Analysis In Patients With Relapsing And Primary Progressive Multiple Sclerosis Actrims Forum 2020
P115 Safety Of Ocrelizumab In Multiple Sclerosis Updated Analysis In Patients With Relapsing And Primary Progressive Multiple Sclerosis Actrims Forum 2020
Request to establish a new Level II HCPCS code to identify ocrelizumab.

Ocrelizumab j code. A10sjl62jy ocrelizumab - uniia10sjl62jy ocrelizumab. G35 MS102 Ocrelizumab Ocrevus is also licensed for primary progressive MS PPMS. It was approved by the US.
HCPCS S9329 Home infusion therapy chemotherapy infusion. Your appointment may take 4-6 hours due to premedication before your infusion as well as 1 hour of monitoring post infusion. JXXXX - Injection ocrelizumab 1 mg.
The denial should be reviewed along with the health insurance plans guidelines to determine what to include. Overall the incidence of adverse events was similar between ocrelizumab and. Subscribe to Codify and get the code details in a flash.
J2350 Injection ocrelizumab 1 mg ICD-10 Diagnosis Code Description G35 Multiple sclerosis CLINICAL EVIDENCE ProvenMedically Necessary Primary Progressive Multiple Sclerosis PPMS The Phase 3 ORATORIO study was a multicenter randomized double-blind placebo-controlled global study. Ocrelizumab also significantly reduced the risk of 24-week confirmed disability progression by 25 vs placebo p00365. J2350 - Injection ocrelizumab 1 mg.
Billing CodeAvailability Information Jcode. Every dose after your first will be given as one. Administrative services professional pharmacy services care coordination and all necessary supplies and equipment drugs and nursing visits coded separately per diem do not use this code with S9330 or S9331 S9379.
Ocrevus 300 mg10 mL single-dose vial. J2350 Injection ocrelizumab 1 mg. Injection ocrelizumab 1 mg.
Applicants suggested language is. 300 mg in 10 ml. Each additional hour List separately in addition to code for primary procedure 99601.
OCREVUS is an infusion therapyalso known as IV therapythat is given through an IV placed in your arm administered by a healthcare professional 2 times a year. Listing of a code in this policy does not imply that the service described by the code is a covered or non- covered health service. Ocrelizumab was also superior to placebo in a number of secondary endpoints.
For example at 120 weeks 389 of individuals on ocrelizumab and 551 on the placebo had declined on the time required to walk 25 feet measured using the Timed 25-Foot Walk test. Intravenous infusion for therapy prophylaxis or diagnosis specify substance or drug. JXXXX - Injection ocrelizumab 1 mg.
Request a Demo 14 Day Free Trial Buy Now. Also other improvements with ocrelizumab were seen in imaging measures like lesion. HCPCS Code for Injection ocrelizumab 1 mg J2350 HCPCS code J2350 for Injection ocrelizumab 1 mg as maintained by CMS falls under Drugs Administered by Injection.
J2350 - Injection ocrelizumab 1 mg. Initial up to 1 hour. It is a humanized anti-CD20 monoclonal antibody.
Intravenous infusion for therapy prophylaxis or diagnosis specify substance or drug. Applicants suggested language is. 1 mg 1 billable unit NDC.
If your patients health insurance plan has issued a denial your Clinical and Reimbursement Manager CRM or OCREVUS Patient Navigator can provide resources as you prepare an appeal submission as per your patients plan requirements. 1 mg 1 billable unit NDC. Code Ocrelizumab Ocrevus is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis RMS with active disease defined by clinical or imaging features.
This protocol is for the RMS indication only. Drugs administered other than oral method chemotherapy drugs. HCPCS J2350 Injection ocrelizumab 1 mg Home infusion.
Food and Drug Administration in March. J2350 is a valid 2021 HCPCS code for Injection ocrelizumab 1. Ocrevus 300 mg10 mL single-dose vial.
It targets CD20 marker on B lymphocytes and hence is an immunosuppressive drug. South San Francisco CA. Billing CodeAvailability Information HCPCS.
Request to establish a new Level II HCPCS code to identify ocrelizumab. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
 Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
 Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
Https Www Hca Wa Gov Assets Billers And Providers Multiple Sclerosis Agents Ocrelizumab Pdf
 Ocrelizumab In Relapsing Remitting Multiple Sclerosis A Phase 2 Randomised Placebo Controlled Multicentre Trial The Lancet
Ocrelizumab In Relapsing Remitting Multiple Sclerosis A Phase 2 Randomised Placebo Controlled Multicentre Trial The Lancet
Https Www Modahealth Com Pdfs Med Criteria Ocrevus Pdf
Https Www Uhcprovider Com Content Dam Provider Docs Public Policies Comm Medical Drug Ocrevus Ocrelizumab Pdf
 Ocrevus Fda Prescribing Information Side Effects And Uses
Ocrevus Fda Prescribing Information Side Effects And Uses
 Tysabri Has About 8 Claims Per Patient Per Year Compared To Lemtrada Which Has 3 And Ocrevus Which Has 2 For Multiple Sclerosis Patients
Tysabri Has About 8 Claims Per Patient Per Year Compared To Lemtrada Which Has 3 And Ocrevus Which Has 2 For Multiple Sclerosis Patients
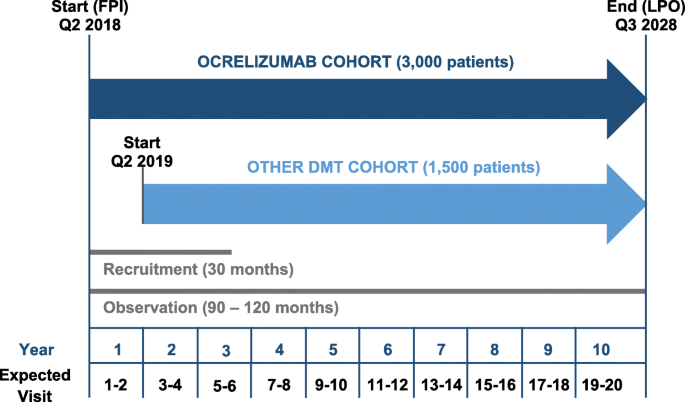 Design Of A Non Interventional Post Marketing Study To Assess The Long Term Safety And Effectiveness Of Ocrelizumab In German Real World Multiple Sclerosis Cohorts The Confidence Study Protocol Bmc Neurology Full
Design Of A Non Interventional Post Marketing Study To Assess The Long Term Safety And Effectiveness Of Ocrelizumab In German Real World Multiple Sclerosis Cohorts The Confidence Study Protocol Bmc Neurology Full
 The Ocrelizumab Phase Ii Extension Trial Suggests The Potential To Improve The Risk Benefit Balance In Multiple Sclerosis Medrxiv
The Ocrelizumab Phase Ii Extension Trial Suggests The Potential To Improve The Risk Benefit Balance In Multiple Sclerosis Medrxiv
Https Www Gatewayhealthplan Com Portals 0 Med Drug Policies Mp 021 Mc Pa Ocrevusoct19 Pdf
 P064 Long Term Reduction Of Relapse Rate And Confirmed Disability Progression After 6 Years Of Ocrelizumab Treatment In Patients With Relapsing Ms Actrims Forum 2020
P064 Long Term Reduction Of Relapse Rate And Confirmed Disability Progression After 6 Years Of Ocrelizumab Treatment In Patients With Relapsing Ms Actrims Forum 2020
The Case For More Trials With Ocrelizumab Can We Use Reduced Dosing Of Ocrelizumab Part Ii The Ms Blog
 Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders
Covid 19 In Ocrelizumab Treated People With Multiple Sclerosis Multiple Sclerosis And Related Disorders

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.