Many labs can perform this test and provide reliable and extremely helpful results. The patient perspective on MRD.
 Clonoseq Report Clonoseq Tracking Mrd Report
Clonoseq Report Clonoseq Tracking Mrd Report
ClonoSEQ pronounced clo-no-seek is the first and only FDA-cleared test that detects counts and tracks MRD in blood or bone marrow samples from patients with chronic lymphocytic leukemia CLL and bone marrow samples from patients with multiple myeloma or.

Clonoseq mrd test. Peer-reviewed real-world evidence analysis. Several US-based cancer centers have proposed care pathways to leverage NGS MRD assessment to support shared decisions around timing of treatment discontinuation for myeloma patients who have been on indefinite maintenance. The clonoSEQ Assay was granted de novo designation by the FDA and is the only MRD assessment tool to have received FDA clearance for the measurement of MRD in patients with B-Cell ALL or MM3.
Distribution of timepoints at which MRD is monitored using the clonoSEQ Assay in lymphoid malignancy patients in real world settings Time Frame. ClonoSEQ is available as an FDA-cleared in vitro diagnostic IVD test service provided by Adaptive Biotechnologies to detect measurable residual disease MRD in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia B-ALL and blood or bone marrow from patients with chronic lymphocytic leukemia CLL. The clonoSEQ Assay measures minimal residual disease MRD to monitor changes in burden of disease during and after treatment.
It is the first and only test to be FDA-approved for MRD. ClonoSEQ is also available for use in other. The clonoSEQ assay is the first and only FDA-cleared in vitro diagnostic for MRD monitoring in CLL.
ClonoSEQ is available as an FDA-cleared in vitro diagnostic IVD test service provided by Adaptive Biotechnologies to detect minimal residual disease MRD in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia B-ALL and blood or bone marrow from patients with chronic lymphocytic leukemia CLL. The clonoSEQ Assay measures minimal residual disease MRD to monitor changes in burden of disease during and after treatment. Test Your clonoSEQ Knowledge.
The test is indicated for use by qualified healthcare professionals in accordance with professional guidelines for clinical decision-making and in. Belova59Pixabay Adaptive Biotechnologies has secured an expanded US Food and Drug Administration FDA approval for its clonoSEQ Assay to detect and monitor minimal residual disease MRD in patients with chronic lymphocytic leukemia CLL. The test is indicated for use by qualified healthcare professionals in accordance with professional guidelines for clinical decision-making and in conjunction with other clinicopathological features.
Observations and analysis of real-world clonoSEQ results. Leading expert views on the role of MRD in patient care. Up to 3 yrs Data will be collected to determine at what points lymphoid malignancy patients are in their treatment continuums when the clonoSEQ Assay is used to monitor MRD levels.
ClonoSEQ Assay will measure MRD in CLL patients. Flow cytometry is the standard test to look for Measurable Minimal Residual Disease MRD in CLL and it can find one cancer cell in 10000 by looking at the telltale combination of surface markers that are found on CLL cells but not on normal cells. This adds to the existing uses of clonoSEQ as.
The clonoSEQ Assay measures minimal residual disease MRD to monitor changes in burden of disease during and after treatment. NGS MRD clonoSEQ Assay. The clonoSEQ test by Adaptive Biotechnologies is being used by doctors to determine how much myeloma a patient still might have following treatment.
The test is indicated for use by qualified healthcare professionals in accordance with professional guidelines for clinical decision-making and in conjunction with other clinicopathological features. When experience builds to evidence. Adaptive Biotechnologies is currently the only FDA cleared MRD test available for patients with MM using bone marrow samples.
The FDA cleared the clonoSEQ assay to detect and monitor minimal residual disease MRD in blood or bone marrow from patients with chronic lymphocytic leukemia CLL according to Adaptive Biotechnologies the developer of the assay. The FDA has cleared the clonoSEQ assay to identify and monitor minimal residual disease MRD in blood or bone marrow from patients with chronic lymphocytic leukemia CLL according to an. Pairwise comparison of MRD frequency measurements from multiparametric flow cytometry mpFC.
The test uses Next Generation Sequencing NGS technology to assess disease burden. The first MRD test in multiple myeloma has now been approved by the FDA. Food and Drug Administration permitted marketing of ClonoSEQ assay a next generation sequencing NGS-based test for minimal residual disease MRD in patients with acute.
The test is indicated for use by qualified healthcare professionals in. ClonoSEQ is also available for use in other. X-axis and the clonoSEQ Assay y-axis for ALL and MM.
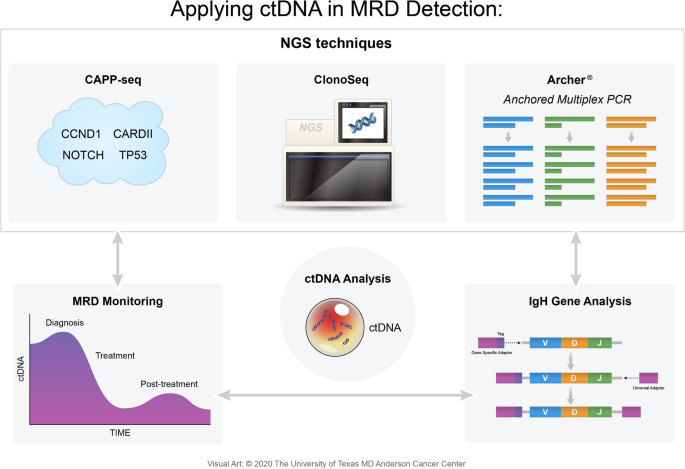 Advances In The Assessment Of Minimal Residual Disease In Mantle Cell Lymphoma Journal Of Hematology Oncology Full Text
Advances In The Assessment Of Minimal Residual Disease In Mantle Cell Lymphoma Journal Of Hematology Oncology Full Text
 Clonoseq Report Clonoseq Tracking Mrd Report
Clonoseq Report Clonoseq Tracking Mrd Report
 Fda Gewahrt De Novo Bezeichnung Fur Clonoseq Assay Von Adaptive Biotechnologie Zur Erkennung Und Uberwachung Von Minimaler Resterkrankung Minimal Residual Disease Mrd Bei Patienten Mit Business Wire
Fda Gewahrt De Novo Bezeichnung Fur Clonoseq Assay Von Adaptive Biotechnologie Zur Erkennung Und Uberwachung Von Minimaler Resterkrankung Minimal Residual Disease Mrd Bei Patienten Mit Business Wire
 Clonoseq Report Clonoseq Tracking Mrd Report
Clonoseq Report Clonoseq Tracking Mrd Report
 Clinical Data Clonoseq Mrd Detection Monitoring For Lymphoid Malignancies
Clinical Data Clonoseq Mrd Detection Monitoring For Lymphoid Malignancies
 Pdf Analytical Evaluation Of The Clonoseq Assay For Establishing Measurable Minimal Residual Disease In Acute Lymphoblastic Leukemia Chronic Lymphocytic Leukemia And Multiple Myeloma
Pdf Analytical Evaluation Of The Clonoseq Assay For Establishing Measurable Minimal Residual Disease In Acute Lymphoblastic Leukemia Chronic Lymphocytic Leukemia And Multiple Myeloma
 Adaptive Technologies Clonoseq Reg
Adaptive Technologies Clonoseq Reg
 Fda Gewahrt De Novo Bezeichnung Fur Clonoseq Assay Von Adaptive Biotechnologie Zur Erkennung Und Uberwachung Von Minimaler Resterkrankung Minimal Residual Disease Mrd Bei Patienten Mit Business Wire
Fda Gewahrt De Novo Bezeichnung Fur Clonoseq Assay Von Adaptive Biotechnologie Zur Erkennung Und Uberwachung Von Minimaler Resterkrankung Minimal Residual Disease Mrd Bei Patienten Mit Business Wire
 Clonoseq On Twitter It S A Great Day For Mm And All Patients Us Fda Cleared Adaptive S Test To Assess Minimal Residual Disease In Bone Marrow From Myeloma Or B Cell All Patients For Important
Clonoseq On Twitter It S A Great Day For Mm And All Patients Us Fda Cleared Adaptive S Test To Assess Minimal Residual Disease In Bone Marrow From Myeloma Or B Cell All Patients For Important
 Mrd Testing With Clonoseq Know Your Residual Disease Status How To Gain Confidence Best Doctors Cancer Journey
Mrd Testing With Clonoseq Know Your Residual Disease Status How To Gain Confidence Best Doctors Cancer Journey
 Abbvie To Deploy Adaptive S Clonoseq Assay To Assess Mrd Status Across Multiple Myeloma Studies Pharmashots
Abbvie To Deploy Adaptive S Clonoseq Assay To Assess Mrd Status Across Multiple Myeloma Studies Pharmashots
 Clonoseq Report Clonoseq Tracking Mrd Report
Clonoseq Report Clonoseq Tracking Mrd Report
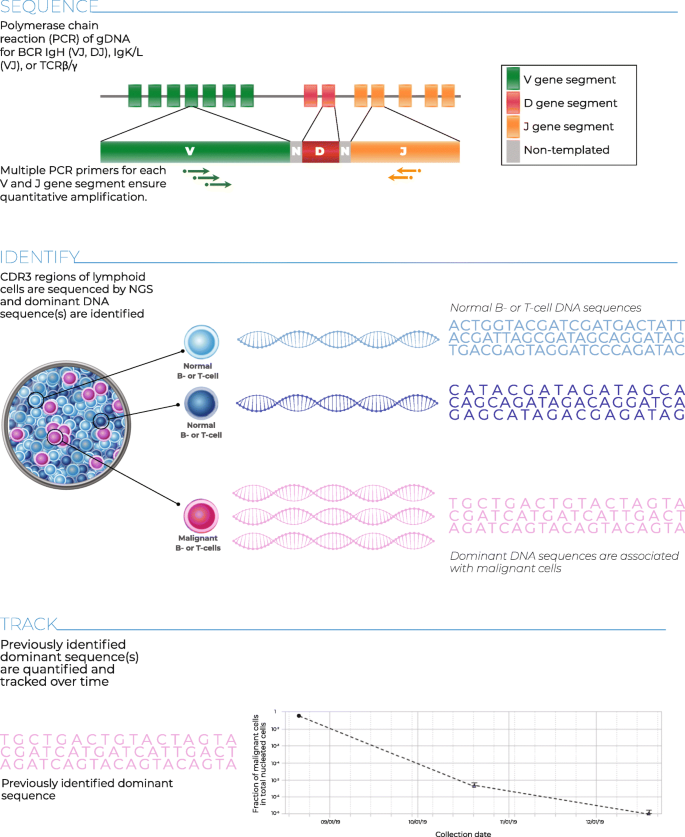 Analytical Evaluation Of The Clonoseq Assay For Establishing Measurable Minimal Residual Disease In Acute Lymphoblastic Leukemia Chronic Lymphocytic Leukemia And Multiple Myeloma Bmc Cancer Full Text
Analytical Evaluation Of The Clonoseq Assay For Establishing Measurable Minimal Residual Disease In Acute Lymphoblastic Leukemia Chronic Lymphocytic Leukemia And Multiple Myeloma Bmc Cancer Full Text
 Clonoseq Report Clonoseq Tracking Mrd Report
Clonoseq Report Clonoseq Tracking Mrd Report

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.