RENFLEXIS Infliximab Powder for Injection CAS number. Each RENFLEXIS vial contains 100.

An unusual viral infection called cytomegalovirus infection.
Renflexis j code. RENFLEXIS is intended for use under the guidance and supervision of a physician. Willst du also z. Renflexis or Remicade will be required to change therapy to Inflectra infliximab -dyyb or Avsola infliximab -axxq unless they meet the criteria in this section.
RENFLEXIS safely and effectively. Find out about insurance coverage for RENFLEXIS including any out-of-pocket costs to you. Reason For Use RFU Code and Clinical Criteria A.
HCPCS Code Details - J1745. Rheumatoid Arthritis Code 541 For the treatment of rheumatoid arthritis RA in patients who have severe active disease greater than or equal to 5 swollen joints and rheumatoid factor positive andor anti-CCP. You should be sure to bill 10 units of J1745 on the claim form when indicating that a single 100-mg vial of REMICADE was used.
Mit deinem Galaxy S10 einen QR-Code scannen gehe wie folgt vor. To speak with a representative from The Merck Access Program call 866-847-3539 Monday to Friday 8 AM to 8 PM Eastern Time. SERIOUS INFECTIONS and MALIGNANCY See full prescribing information for complete boxed warning.
RENFLEXIS infliximab-abda for Injection for Intravenous Use. Infection due to Nocardia bacteria. What is your patients current weight.
RENFLEXIS infliximab-abda is biosimilar to REMICADE infliximab. What are the Limited Use Criteria for Renflexis infliximab. A type of.
Very rare less than 001. Coverage for Renflexis infliximab-abda Remicade infliximab or other non-preferred infliximab product will be provided contingent on the criteria in this section and the coverage criteria in the. Drugs administered other than oral method chemotherapy drugs J1745 is a valid 2021 HCPCS code for Injection infliximab excludes biosimilar 10 mg or just Infliximab not biosimil 10mg for short used in Medical care.
Q5104 is a valid 2021 HCPCS code for Injection infliximab-abda biosimilar renflexis 10 mg or just Injection renflexis for short used in Medical care. HU United States Minor Outlying Islands. HCPCS Level II Code Drugs administered other than.
The product-specific HCPCS code for REMICADE is J1745 infliximab 10 mg. In order to continue. RENFLEXIS Infliximab.
UZ Land ISO 3166 ALPHA-2 ISO 3166 ALPHA-3 ISO 3166 numerisch TLD IOC ISO 3166-2 UN Ortscode. It is important to note that this code represents 110th of a vial. A systemic inflammatory response called sepsis due to an infection with bacteria.
TE Code RLD RS. Q5104 Injection infliximab-abda biosimilar renflexis 10 mg Q2041 Axicabtagene Ciloleucel up to 200 million autologous Anti-CD19 CAR T Cells including leukapheresis and dose preparation procedures per infusion. Injection infliximab excludes biosimilar 10 mg.
Renflexis which is a proposed biosimilar to US-licensed Remicade also referred to as US-Remicade in this review with the active ingredient infliximab a TNF. Proposed trade name. Adult patients with active disease when the.
Retrobulbar optic neuritis of the left eye orbital cellulitis third nerve palsy transient visual loss associated with infliximab the active ingredient contained in Renflexis administration during or within 2 hours of infusion. NAME OF THE MEDICINE. Immunosuppressants tumor necrosis factor alpha TNF-α inhibitors L04AB02 Therapeutic indications.
The comparability of RENFLEXIS with Remicade has been demonstrated with regard to. Rheumatoid arthritis Flixabi in combination with methotrexate is indicated for the reduction of signs and symptoms as well as the improvement in physical function in. The reconstituted infusion solution should be prepared by a trained medical professional using aseptic technique by the following procedure.
See full prescribing information for RENFLEXIS. Avsola 100mg vial Inflectra 100mg vial Renflexis 100mg vial Other please specify. Learn about options for co-pay assistance for eligible privately-insured patients.
1 Drücke zweimal kurz hintereinander die EinAus-Taste um die Kamera deines S10 zu starten und richte die Kamera auf den QR-Code. Frequency of administration. RENFLEXIS Version 60 Page 1 62.
Das Galaxy S10 oder das Tab S6 haben den QR-Code Scanner direkt in der Statusleiste integriert. Preferred Product Criteria Treatment with Remicade Renflexis or other infliximab biosimilar product is medically necessary for the indications. RENFLEXIS infliximab is a biosimilar medicine to Remicade infliximab.
1 vial 10 units. Calculate the dose total volume of reconstituted RENFLEXIS solution required and the number of RENFLEXIS vials needed.
Https Www Uhcprovider Com Content Dam Provider Docs Public Policies Index Oxford Infliximab Ohp 020120 Pdf
 Renflexis Infliximab Abda Coding And Billing Information
Renflexis Infliximab Abda Coding And Billing Information
 Drug Channels Remicade A Case Study In How U S Pricing And Reimbursement Curb Adoption Of Biosimilars
Drug Channels Remicade A Case Study In How U S Pricing And Reimbursement Curb Adoption Of Biosimilars
Https Www Merckaccessprogram Renflexis Com Static Pdf Us Sbt 00482 Renflexis Coding And Billing Guide Pdf
Https Www Merckaccessprogram Renflexis Com Static Pdf Us Sbt 00482 Renflexis Coding And Billing Guide Pdf
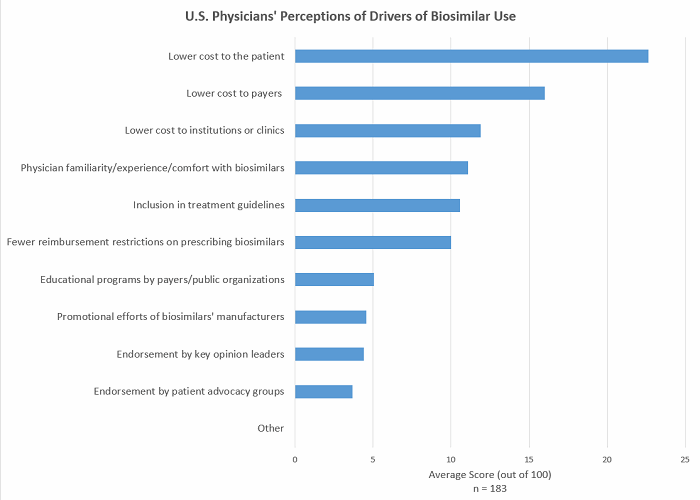 Cms Decision On Coding For Biosimilars A Deeper Look
Cms Decision On Coding For Biosimilars A Deeper Look
 Renflexis Fda Prescribing Information Side Effects And Uses
Renflexis Fda Prescribing Information Side Effects And Uses
Https Www Merckaccessprogram Renflexis Com Static Pdf Us Sbt 00482 Renflexis Coding And Billing Guide Pdf
Https Www Merckaccessprogram Renflexis Com Static Pdf Us Sbt 00482 Renflexis Coding And Billing Guide Pdf
Https Www Merckaccessprogram Renflexis Com Static Pdf Us Sbt 00482 Renflexis Coding And Billing Guide Pdf
 Infliximab Test Now Validated For Renflexis Insights
Infliximab Test Now Validated For Renflexis Insights
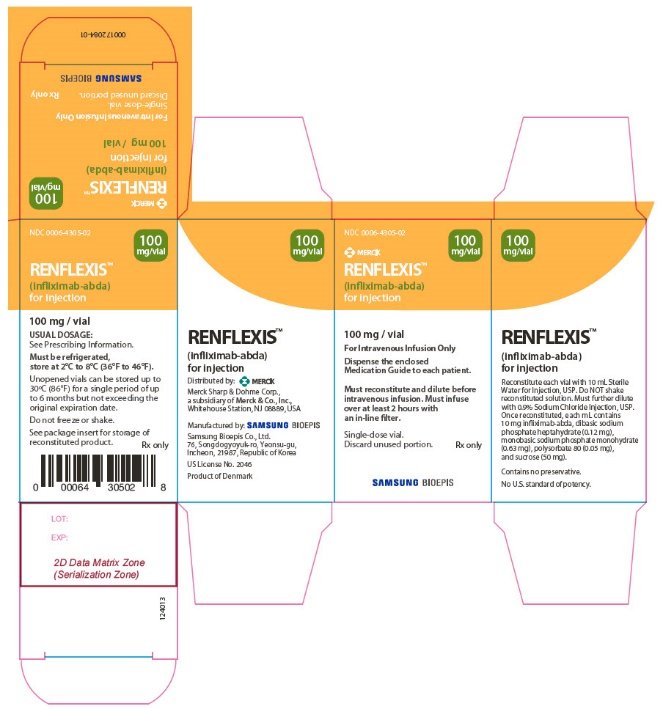
 Ndc 0006 4305 Renflexis Infliximab
Ndc 0006 4305 Renflexis Infliximab

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.